Abstract
Background: An immune suppressive tumor microenvironment (TME) plays a significant role in the pathogenesis and progression of T-cell lymphomas (TCLs). Therapies that reverse the suppressive TME or increase tumor antigen presentation could be additive or synergistic with immunotherapeutic strategies. One concern with combining cytotoxic chemotherapy (chemo) with immunotherapy such as checkpoint blockade (CPB) is that it could reduce or eliminate anti-tumor effector cells, thus reducing efficacy. We performed a phase I clinical trial using the immune CPB nivolumab (Nivo) in combination with DA-EPOCH in newly diagnosed peripheral T-cell lymphomas (Haverkos et al., ASH. 2021) and studied the T-cell phenotype and function at baseline, during therapy, and at end of treatment (EOT) of patients (pts) receiving CPB + chemo compared to contemporaneous cohorts of TCL pts treated with chemo or CPB alone outside the context of a clinical trial.
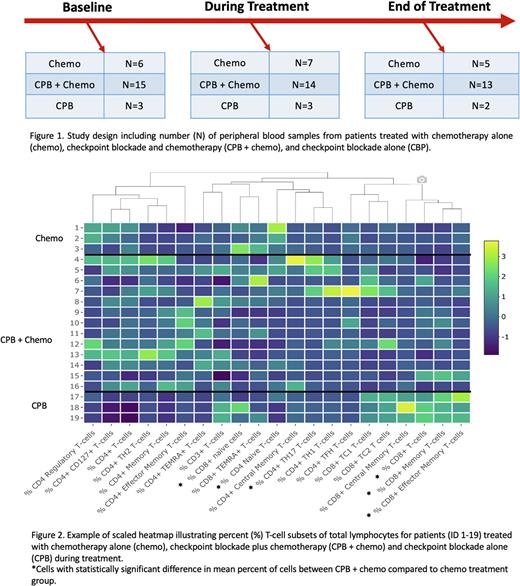
Methods: Peripheral blood was collected serially at baseline, during treatment, and end of treatment (EOT) in TCL pts treated on clinical trial with Nivo + DA-EPOCH, standard of care chemo, and single agent CPB (Figure 1). We isolated mononuclear cells and performed multi-color flow cytometry using three validated flow panels: T-cell Phenotype, T-cell Activation/Exhaustion, and Lymphocyte Cytokine. For the Phenotype Panel, T-cells were categorized as naïve (CD45RA+ CCR7+), central memory (CD45RA- CCR7+), effector memory (CD45RA- CCR7-) helper (CXCR3+, CCR4+, or CCR6+) and regulatory (FoxP3+). For the Activation/Exhaustion Panel, samples were stained with antibodies to identify expression of inhibitory receptors (TIM3, LAG3, PD-1), activation markers (CTLA-4), and effector cell transcription factors (T-bet, Eomes). For the Cytokine Panel, cells were stimulated with PMA/Ionomycin and cytokines including interferon-γ, TNFα, IL2, IL4, IL17, and granzyme B were measured. At each timepoint, we visualized differences in T-cell subset percents (%) of total lymphocytes between treatment cohorts using heat maps. We then tested differences in mean % of cells between treatment groups during treatment and at EOT using linear regression. Given limited sample size, differences where p<0.1 are being further studied using single cell and targeted RNA sequencing (RNAseq). Additionally, we are testing mean % changes within each cohort between timepoints.
Results: First, we compared CPB + chemo vs. chemo alone cohorts using our three flow panels. With respect to cell Phenotype Panel, pts treated with CPB + chemo had higher % of total CD8+ (p=0.046), CD8+ memory (p=0.049), and CD4+ effector memory T-cells (p=0.059) during treatment (Figure 2). There were lower % of CD4+ and CD8+ naïve T-cells (p<0.001 and p=0.032, respectively) and CD4+ helper T-cells (p=0.058) during treatment. Higher % of CD8+ memory cells (p=0.030) and lower CD8+ naïve cells (p=0.013) persisted at EOT. There was no difference in the overall % of CD4+ cells during treatment or EOT (p=0.137 and p=0.535). Using the cell Activation/Exhaustion Panel, we identified lower % of CD4+ and CD8+ CTLA4+ memory cells (p=0.010 and p=0.013, respectively), CD8+ Eomes+ memory cells (p=0.013), CD4+ and CD8+ TIM3+ memory cells (p=0.042 and p=0.084, respectively), CD4+ PD1+ naïve cells (p=0.038), and CD4+ and CD8+ PD1+ memory cells (p=0.002 and p=0.003, respectively) during treatment. There were no statistically significant differences in activation/exhaustion markers at EOT. Lastly, the Cytokine Panel demonstrated higher % of CD8+ granzyme B positive T-cells during treatment (p=0.039) but no differences at EOT. Differences in cell makeup in the CPB only group (N=2) were similar to the changes in CPB + chemo group. Further validation of these findings by evaluating serial changes in T-cell subsets within each cohort and RNAseq is ongoing.
Conclusions: Our results suggest that with the addition of CPB, CD8+ T-cells (as a % of all lymphocytes) increase initially, but there is a transition in cell phenotype during treatment. The combination of CPB + chemo may initially lead to an increase in anti-tumor immune effector cells, but these benefits could be temporary when chemo is continued for >2 cycles. Similarly, in our Nivo + DA-EPOCH clinical trial, we observed early initial clinical responses that did not persist. This data should be considered during development of future chemoimmunotherapeutic treatment strategies.
Disclosures
Zain:Secura Bio: Consultancy, Research Funding; Daichi Sankyo: Consultancy, Research Funding; AstraZeneca: Research Funding; Myeloid: Consultancy, Research Funding; CRSPR: Research Funding; Seattle Genetics: Research Funding, Speakers Bureau; 3M: Current holder of stock options in a privately-held company; Affirmed: Research Funding; Kiyowa Kirin: Speakers Bureau. Porcu:Ono: Membership on an entity's Board of Directors or advisory committees; ADCT: Membership on an entity's Board of Directors or advisory committees; Loxo: Membership on an entity's Board of Directors or advisory committees; Daiichi, Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; DrenBio: Consultancy; Teva: Honoraria, Research Funding; Viracta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Haverkos:Viracta Therapeutics: Consultancy; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal